Biotechnologies
Innovative Point-of-Care Testing Platform using BAW Technology
Qorvo Biotechnologies is developing a point-of-care (POC) diagnostic test platform that aims to change how healthcare is provided. Our system is based on high performance bulk acoustic wave (BAW) detection, designed with the goal to provide accurate test results in approximately 20 minutes.
Download our White Papers:
- Qorvo Biotechnologies Omnia™ SARS-CoV-2 Antigen Test Detects Delta and Other Circulating COVID-19 Variants
- Qorvo Biotechnologies Omnia™ Instrument and SARS-CoV-2
Antigen Test Completes Independent Verification from the National
Institute of Health (NIH) as Part of the Rapid Acceleration of
Diagnostics (RADx) Initiative
Please visit QorvoBiotech.com for more detailed information.


BAW enables surface-based mass measurement. Qorvo’s BAW technology uses high frequency and surface binding that, based on results of early stage feasibility studies, suggests the potential for sensitivity and variance control similar to central labs. These two factors have limited the ubiquitous deployment of point-of-care solutions based on historical optical or fluorescence detection systems. The innovation is uniquely positioned to allow for the dual testing capability of Molecular and Immunoassays on the same cartridge and instrument interface. Beyond this, BAW allows for multiplexing (multiple analytes tested off a single sample), and the cartridge has been designed to allow measurements of multiple matrix types including whole blood, serum, plasma, saliva and more. The goal is to enable clinicians’ access to point-of-care test data in approximately 20 minutes.
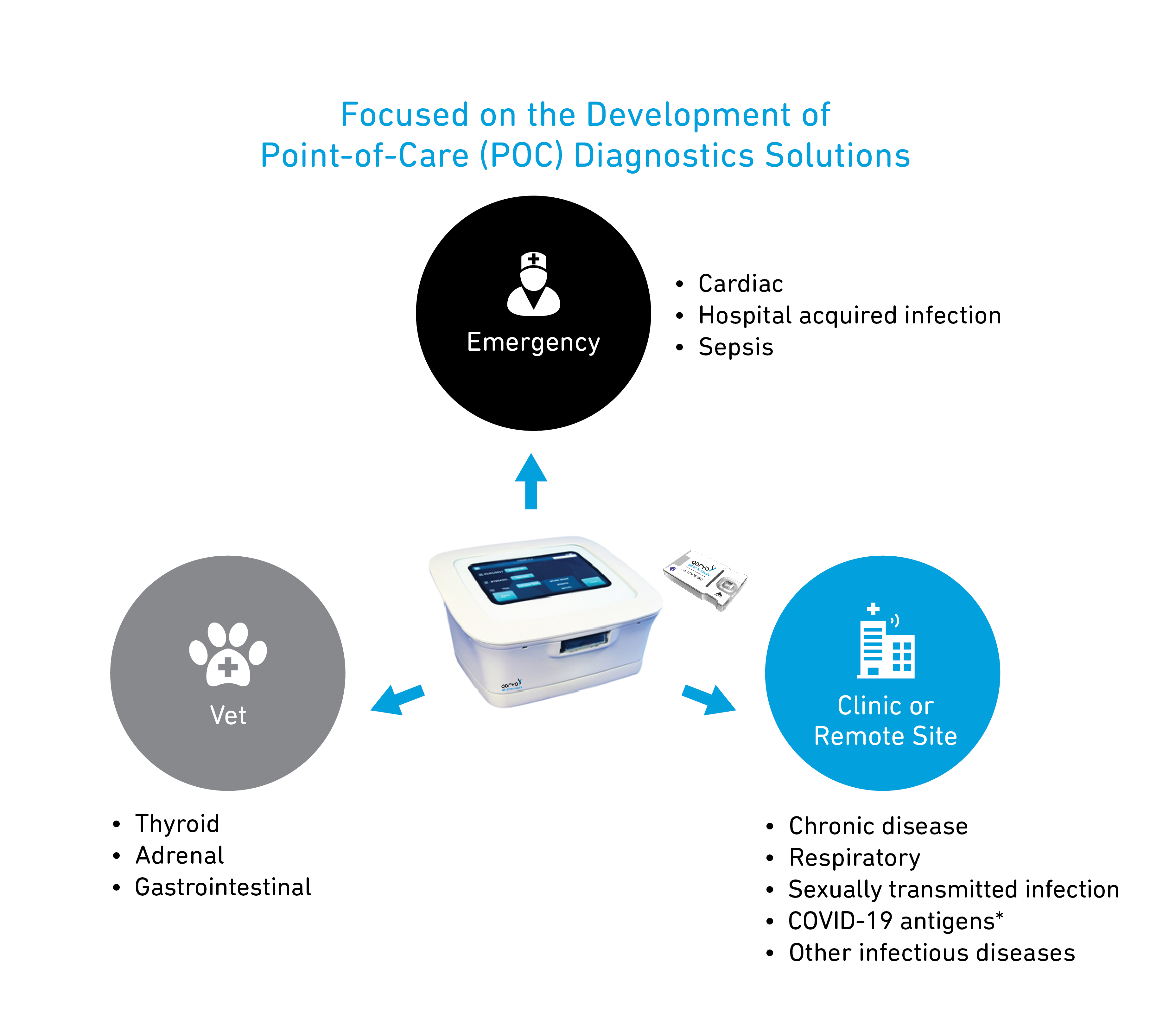
*This product has not been FDA cleared or approved, but has been authorized by FDA under an EUA for use by authorized laboratories. This product has been authorized only for the detection of proteins from SARS-CoV-2, not for any other viruses or pathogens; and the emergency use of this product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug and Cosmetic Act, 21 U.S.C. § 360bbb-3(b)(1), unless the declaration is terminated or authorization is revoked sooner.
